Theoretical Studies on Mechanism of Ru(II)-CatalyzedRegioselective C-H Allylation of Indoles with Allyl Alcohols
Chao Deng,* Po Hu, YouJia Wang, Shaowei Wang and Weihua Zhang*
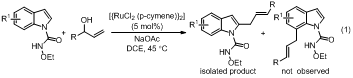
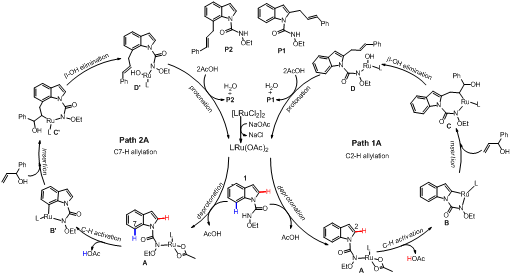
The reaction mechanism of the Ru(II)-catalyzedregioselective C-H allylation of indoles with allyl alcohols has been studied by density functional theory (DFT) calculations. This reaction mechanism involves five major steps: deprotonation of amide-NH,areneC-H activation, allyl alcohol insertion,β-OH elimination and protonation. Our calculation results indicate that the C2-H bond allylation is better than C7-Hbond allylation, which can be attributed to the stronger nucleophilicity of C2 compared to C7 in the C−Rubond insertion of indole substrates. Furthermore, we also suggest the C-Ru bond insertion is more favorable than N-Ru bond insertion, which can be attributed to the different hybridization states of the relevant carbon and nitrogen atoms. Meanwhile,we also illustrate the substrate cinnamyl alcoholcan not give the desired product due to the steric hindrance of phenyl ring in cinnamyl alcohol with indole skeleton.
This paper has been accepted by Dalton Transactions, 2019, DOI: 10.1039/C9DT01674K IF = 4.099, Top 20%.